CD20:狂奔中的“戰馬”,新型藥研發從未停歇!
日期:2022-11-15 15:07:45
近期,CD20靶向療法在自身免疫疾病領域取得顯著突破。羅氏第三代CD20單抗Gazyva(奧妥珠單抗)火力全開:10月獲FDA批準用于狼瘡性腎炎(LN),成為該領域首款CD20靶向療法;隨后在特發性腎病綜合征的INShore試驗中展現優勢,并于11月宣布系統性紅斑狼瘡(SLE)的3期研究達到主要終點。Gazyva通過高效清除B細胞、半年一次的給藥頻率以及良好安全性,正從腫瘤治療向自免領域快速拓展,驗證了成熟靶點在跨疾病應用中的持續潛力。
1. 什么是CD20?
CD20是位于B細胞上的一種非糖基化磷蛋白,主要在前B細胞到成熟B細胞階段表達。CD20分子屬疏水、4次跨膜蛋白,由297個氨基酸殘基組成,相對分子質量(Mr)約為33×103。CD20的表達在不同的B細胞惡性腫瘤之間是高度可變的 [2-3]。CD20的分子功能與B細胞受體(BCR)的信號傳導傾向有關。CD20被證明與B細胞上的其他多種表面蛋白(如CD40、CD53、CD81和CD82)相互作用。有研究表明,一些表觀遺傳因子(EZH2、HDAC1/2、HDAC6、Sin3A-HDAC1復合物)和轉錄因子(USF、OCT1/2、PU.1、PiP、ELK1、ETS1、SP1、NFκB、FOXO1、SMAD2/3)也可調節CD20的表達 [4-6]。大量研究已證實CD20是人類B淋巴細胞表面特異性分子標記物,對B淋巴細胞的增殖和分化具有調節作用。絕大部分的B淋巴細胞瘤都有CD20的表達,CD20分子易與抗體結合,且結合后不易脫落、不內化,成為治療B細胞淋巴瘤的理想靶抗原。目前,抗CD20單克隆抗體是治療B細胞淋巴瘤(B-cell non-Hodgkin’s lymphoma)的重要靶向藥(圖1) [4]!
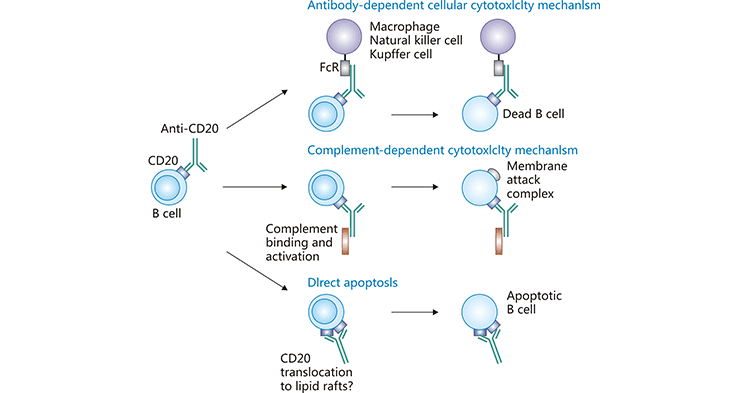
圖1. 抗CD20單克隆抗體是治療BCL的重要靶向藥 [4]
2. CD20相關的調節機制
2.1 CD20與B細胞抗原受體
CD20在B淋巴細胞表面以寡聚體形式存在,現有實驗證明CD20在B細胞表面形成的是四聚體。CD20與B細胞抗原受體(B cell antigen receptor,BCR)的膜型IgM(sIgM)存在相互作用已被被證實,B細胞活化后,BCR-CD20復合物會解離,磷蛋白、鈣調素結合蛋白會暫時被招募到CD20,從而參與胞內信號的傳導 [2-4]。
2.2 CD20與鈣離子信號傳導
鈣離子流動對細胞的生物學功能有重要影響。鈣池可調控鈣離子進人(store-operated calcium entry,SOCE),主要是通過鈣離子釋放激活鈣離子(calcium-release-activatedcalcium,CRAC)通道來提高細胞內的鈣離子濃度,是淋巴細胞提高胞內鈣離子濃度的主要方式。越來越多的證據表明CD20參與了SOCE,CD20對于胞內鈣離子濃度有重要的調節作用,而SOCE所引起鈣離子流的強度和持續時間,將影響受體與抗原結合后細胞的功能 [6-8]。
2.3 抗CD20單抗與B細胞淋巴瘤
抗CD20單克隆抗體可以殺死B細胞來源的腫瘤,大量證據表明,抗CD20單克隆抗體與三種作用機制有關,即抗體依賴性細胞毒性(ADCC)、補體依賴性細胞毒性(CDC)和抗體與CD20分子結合引起的直接效應,包括抑制細胞生長、改變細胞周期和細胞凋亡。ADCC是指涂有抗CD20的B細胞通過一種基于細胞的機制被殺死。CDC是指補體膜攻擊復合物在細胞表面組裝起來。除在正常B細胞中表達外,CD20還在B細胞來源的淋巴瘤、白血病等的腫瘤細胞表達,以及涉及免疫疾病和炎癥疾病的B細胞中表達,所以,CD20抗原成為淋巴癌、白血病和某些自體免疫等疾病治療的目標靶點 (圖2)[3, 9-12]。
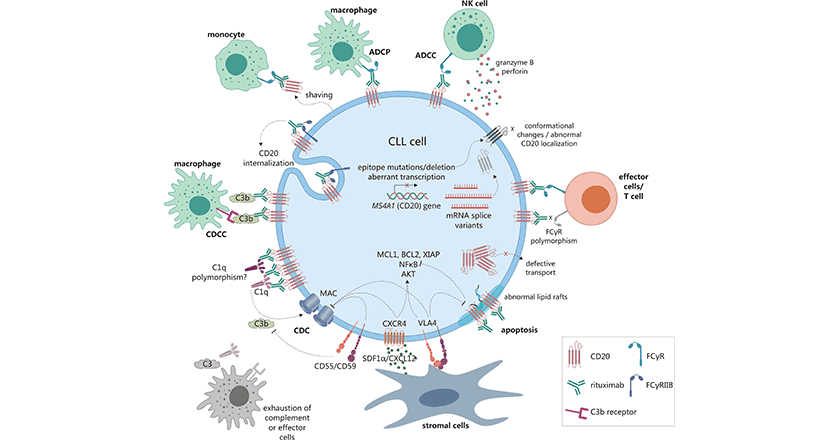
圖2. 抗CD20單抗抗腫瘤調節作用 [3]
3. 靶向CD20的治療方式有哪些?
靶向CD20的治療的藥物,主要有三種形式,抗CD20單克隆抗體、CAR-T和雙特異性抗體。眾多已上市和在研的生物制劑集中在CD20單克隆抗體。根據人源化程度以及Fc片段修飾,抗CD20單抗可分為三代,其中第一代是以利妥昔單抗為代表的嵌合或者鼠源單抗,第二代是以奧法木單抗為代表的人源化單抗,第三代的抗CD20單抗以奧妥珠單抗為代表,其抗體的Fc片段經過了糖基化修飾 [13-15]。
第一代抗CD20治療性單克隆抗體藥利妥昔單抗,臨床多用于聯合治療,特別是難治愈和復發性的淋巴瘤,常用的聯合用藥方式是R-CHOP,即利妥昔單抗、環磷酰胺、阿霉素、長春新堿、和潑尼松聯合使用;第二代抗CD20單抗包括Ofatumumab、Veltuzumab和Ocrelizumab等,降低了第一代抗體的免疫原性,如Ofatumumab能有效誘導利妥昔單抗抵抗的細胞和CD20低表達的惡性B細胞的補體依賴性細胞毒性;第三代單抗奧濱尤妥珠Obinutuzumab,通過糖基化修飾抗體Fc片段,增強靶點結合力,使得ADCC效應增強 [13-15]。
4. 未來展望
CD20靶點的進化遠未止步。羅氏通過三代單抗(利妥昔、奧瑞利珠、奧妥珠)及雙抗技術(如CD20/CD3、CD20/TfR),持續挖掘其價值:在自免領域,Gazyva針對SLE、LN及膜性腎病的臨床研究逐步推進;雙抗平臺更將應用延伸至阿爾茨海默癥等神經系統疾病。結合羅氏近期引進長效自免雙抗等動向,其免疫管線已覆蓋COPD、哮喘、纖維化等多領域。未來,CD20靶點有望通過機制優化與適應癥拓展,成為連接腫瘤、自免及神經疾病的核心橋梁,凸顯“深度耕耘成熟靶點”的創新價值。
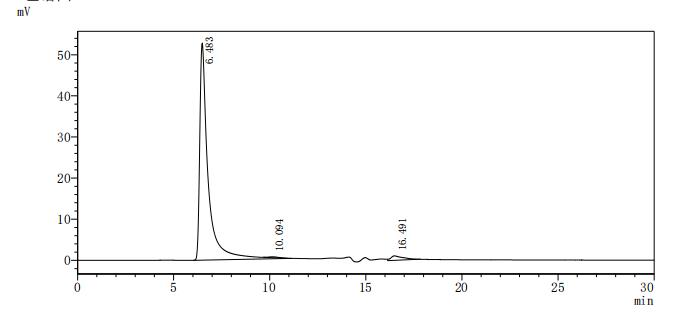
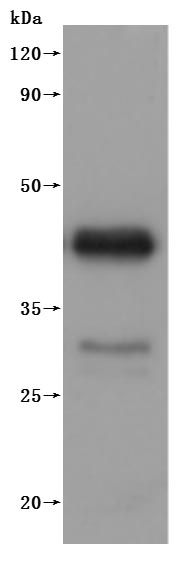
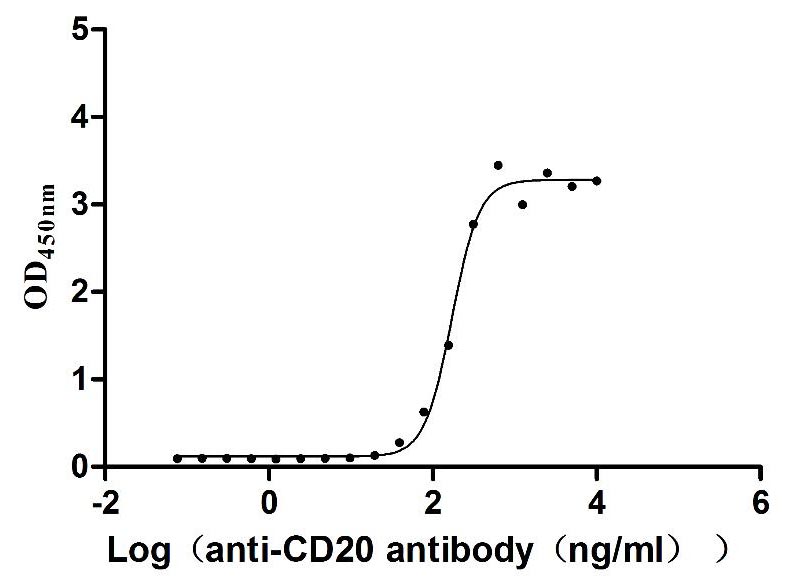
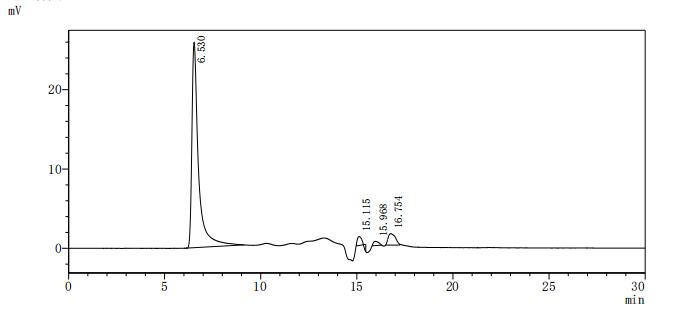
為鼎力協助各藥企針對CD20在淋巴癌、白血病和某些自體免疫等疾病治療領域上的研發,CUSABIO現有CD20活性蛋白產品(Code:CSB-MP015007HU),助力您在CD20機制方面的研究或其潛在臨床價值的探索(點擊查看CD20全系列產品)。
CD20 (MS4A1) 抗體
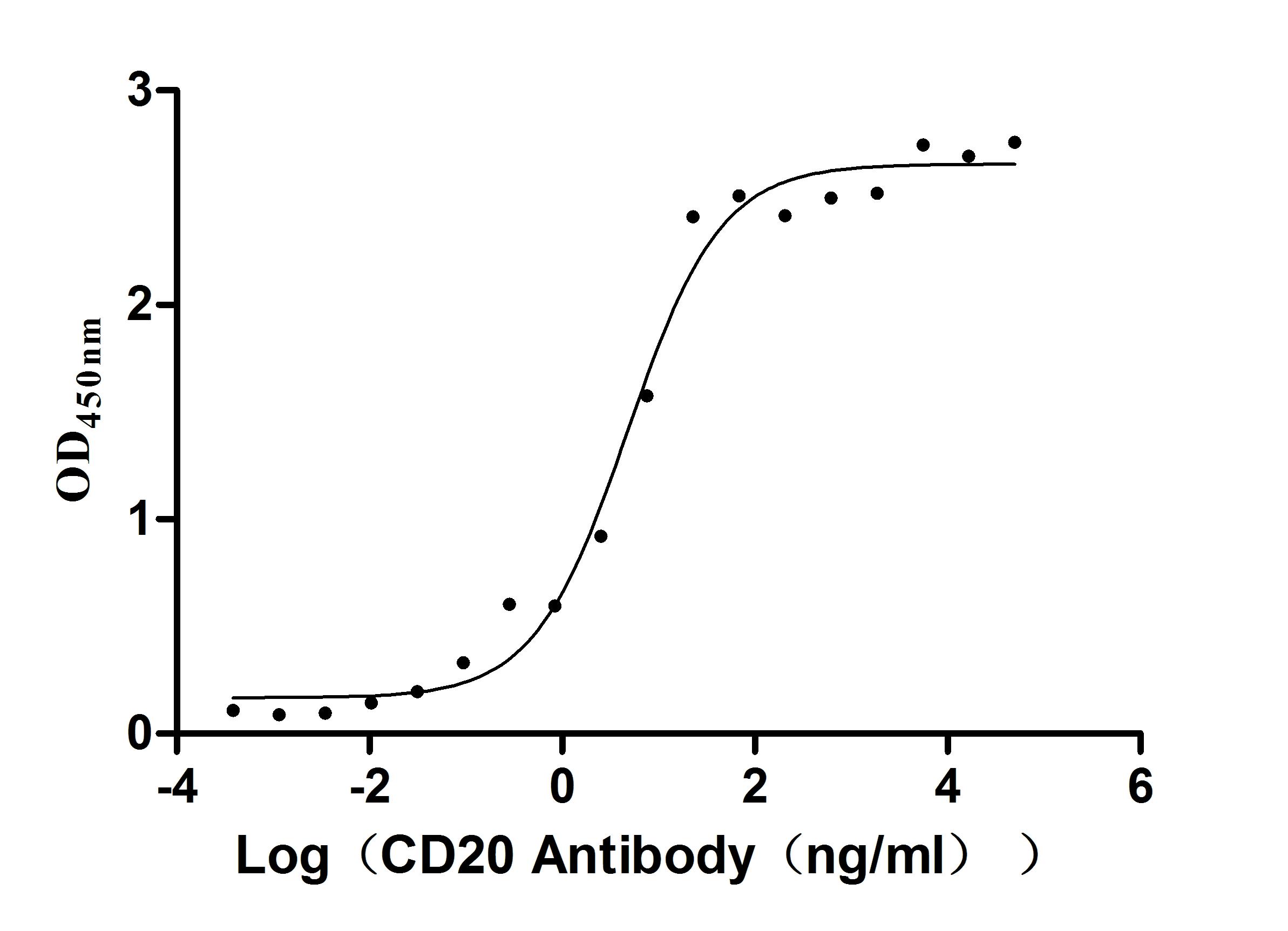
MS4A1 Monoclonal Antibody
(CSB-RA015007A1HU)
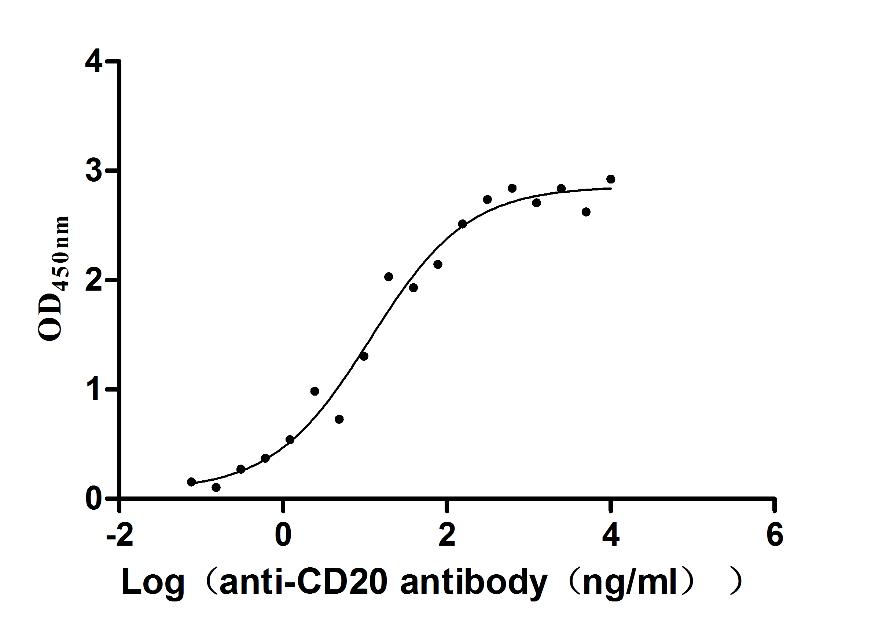
MS4A1 Monoclonal Antibody
(CSB-RA015007MA3HU)
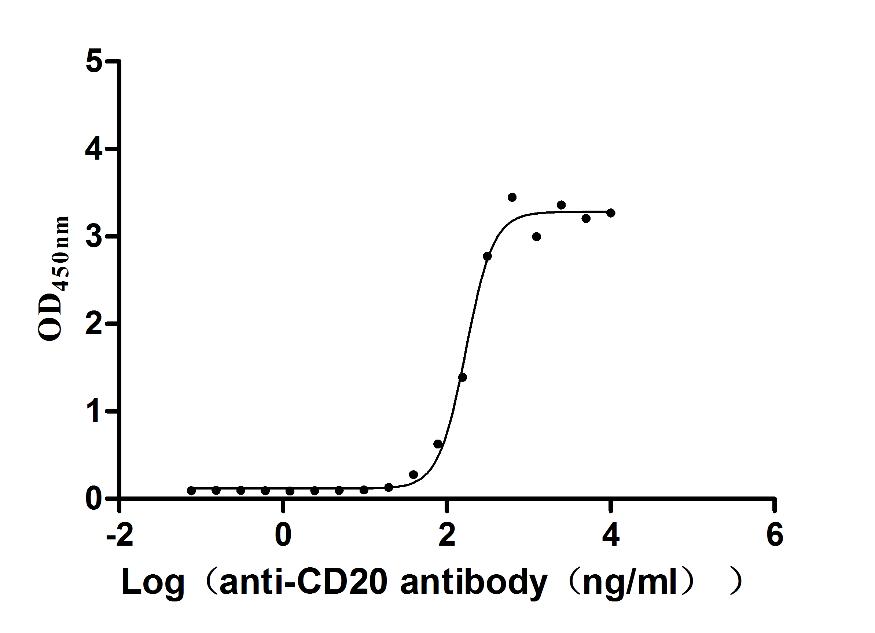
MS4A1 Monoclonal Antibody
(CSB-RA015007MA3HU)
MS4A1 Antibody
(CSB-PA015007DSR2HU)
MS4A1 Antibody
(CSB-PA015007DSR2HU)
MS4A1 Monoclonal Antibody
(CSB-MA000204)
參考文獻:
[1] Shi, Yuankai, et al. "Comparison of efficacy and safety of ripertamab (SCT400) versus rituximab (Mabthera?) in combination with CHOP in patients with previously untreated CD20‐positive diffuse large B‐cell lymphoma: A randomized, single‐blind, phase III clinical trial." Hematological Oncology (2022).
[2] Wu, Xin, et al. "The Efficacy and Safety of Anti-CD20 Antibody Treatments in Relapsing Multiple Sclerosis: A Systematic Review and Network Meta-analysis." CNS drugs (2022): 1-16.
[3] Schilhabel, Anke, et al. "CD20 Expression as a Possible Novel Prognostic Marker in CLL: Application of EuroFlow Standardization Technique and Normalization Procedures in Flow Cytometric Expression Analysis." Cancers 14.19 (2022): 4917.
[4] Pavlasova, Gabriela, and Marek Mraz. "The regulation and function of CD20: an “enigma” of B-cell biology and targeted therapy." Haematologica 105.6 (2020): 1494.
[5] Shree, Tanaya, et al. "CD20-targeted therapy ablates de novo antibody response to vaccination but spares preestablished immunity." Blood cancer discovery 3.2 (2022): 95-102.
[6] Cree, Bruce AC. "All anti-CD20 monoclonal antibodies have similar efficacy and safety risks: Yes." Multiple Sclerosis Journal 28.12 (2022): 1843-1844.
[7] Schuster, Stephen J., et al. "Characterization of CD20 expression loss as a mechanism of resistance to mosunetuzumab in patients with relapsed/refractory B-cell non-Hodgkin lymphomas." (2022): 7526-7526.
[8] Walshe, Claire A., et al. "Induction of cytosolic calcium flux by CD20 is dependent upon B Cell antigen receptor signaling." Journal of Biological Chemistry 283.25 (2008): 16971-16984.
[9] Liu, Jinny L., et al. "Selection and characterization of single domain antibodies against human CD20." Molecular Immunology 78 (2016): 146-154.
[10] Schneider, Dina, et al. "Trispecific CD19-CD20-CD22–targeting duoCAR-T cells eliminate antigen-heterogeneous B cell tumors in preclinical models." Science Translational Medicine 13.586 (2021): eabc6401.
[11] Grandjean, Capucine L., et al. "Imaging the mechanisms of anti-CD20 therapy in vivo uncovers spatiotemporal bottlenecks in antibody-dependent phagocytosis." Science Advances 7.8 (2021): eabd6167.
[12] Pozzo, Federico, et al. "SF3B1-mutated chronic lymphocytic leukemia shows evidence of NOTCH1 pathway activation including CD20 downregulation." haematologica 106.12 (2021): 3125.
[13] Goldenberg, David M., and Robert M. Sharkey. "Sacituzumab govitecan, a novel, third-generation, antibody-drug conjugate (ADC) for cancer therapy." Expert Opinion on Biological Therapy 20.8 (2020): 871-885.
[14] Asano, Teizo, et al. "Epitope mapping of an anti-CD20 monoclonal antibody (C20Mab-60) using the HisMAP method." Monoclonal antibodies in immunodiagnosis and immunotherapy 40.6 (2021): 243-249.
[15] Luo, Chengxin, et al. "Efficacy and safety of new anti-CD20 monoclonal antibodies versus rituximab for induction therapy of CD20+ B-cell non-Hodgkin lymphomas: A systematic review and meta-analysis." Scientific reports 11.1 (2021): 1-14.